Continuing on with the "Anti-Aging Skin Series" -- the time has come to comment on the toxicity of the ingredients in the first product I analyzed -- Jergen's Skin Firming Moisturizer. As you will recall, in the first part of the analysis of the skin firming lotion (Anti-Aging Skin Series Post 5), I introduced you to the sources (EWG, Paula's Choice, and David Suzuki are three examples) of chemical information on where to find the ingredients listed on the back of cosmetic products. This post gave you an idea of the function of the ingredient. In an intermediate post, I decided to compile a short list of a glossary of functions for you to refer to for greater information on each function. Understanding the function of each ingredient gives you a better idea of why the ingredient was needed in the product in the first place.
The next logical post (Part 2) was an exploration of the ingredients in terms of function and also a categorization of the ingredients into the nature of their chemical structure. Those chemicals that possess a greater amount of 'non-polar' character where categorized as 'non-polar'. Whereas, on the other side of the spectrum are molecules which have a higher degree of charge difference in their structure and are therefore classified as 'polar'. In order to bring these two different types of structure into a homogeneous mixture (a cosmetic product that does not separate), an 'emulsifier' is used. As a result of the analysis, in the future products analyzed in this series, you will have a better understanding of the need to incorporate each into a given cosmetic product. The next product I chose to breakdown and analyze is from a claimed "green" company with no harsh chemicals. But before I get to that product, I wanted to speak a little on the toxicity of chemicals found in cosmetic products. Below are a few thoughts and images to drive home the point -- toxicity lies on a spectrum (a range).
What Is Toxicity?
If the valuable resource "Wikipedia" were consulted for an understanding of the meaning 'Toxicity,' the following introduction would be seen below:
Toxicity is the degree to which a substance can damage an organism.[1] Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell (cytotoxicity) or an organ such as the liver (hepatotoxicity). By extension, the word may be metaphorically used to describe toxic effects on larger and more complex groups, such as the family unit or society at large. Sometimes the word is more or less synonymous with poisoning in everyday usage.A central concept of toxicology is that the effects of a toxin are dose-dependent; even water can lead to water intoxication when taken in too high a dose, whereas for even a very toxic substance such as snake venom there is a dose below which there is no detectable toxic effect. Toxicity is species-specific, making cross-species analysis problematic. Newer paradigms and metrics are evolving to bypass animal testing, while maintaining the concept of toxicity endpoints.[2]
The toxicity of a given substance is dependent on the damage done to a given organism or plant. Damage can be broken down into substructures of organisms or plants -- specific parts (organs, metabolic pathways, etc.). That is where the definition gets difficult. Further, toxicity depends on dose -- i.e., how much of the substance was administered? Routes of exposure are just as critical as the amount of substance given. These are just a couple of the main considerations to think about when assessing the toxicity associated with a given chemical.
Next, the type of toxic entity is important to consider in a given assessment. Below are the four types of toxic entities taken from "Wikipedia":
- Chemical toxicants include inorganic substances such as, lead, mercury, hydrofluoric acid, and chlorine gas, and organic compounds such as methyl alcohol, most medications, and poisons from living things. While some weakly radioactive substances, such as uranium, are also chemical toxicants, more strongly radioactive materials like radium are not, their harmful effects (radiation poisoning) being caused by the ionizing radiation produced by the substance rather than chemical interactions with the substance itself.
- Disease-causing microorganisms and parasites are toxic in a broad sense, but are generally called pathogens rather than toxicants. The biological toxicity of pathogens can be difficult to measure because the "threshold dose" may be a single organism. Theoretically one virus, bacterium or worm can reproduce to cause a serious infection. However, in a host with an intact immune system the inherent toxicity of the organism is balanced by the host's ability to fight back; the effective toxicity is then a combination of both parts of the relationship. In some cases, e.g. cholera, the disease is chiefly caused by a nonliving substance secreted by the organism, rather than the organism itself. Such nonliving biological toxicants are generally called toxins if produced by a microorganism, plant, or fungus, and venoms if produced by an animal.
- Physical toxicants are substances that, due to their physical nature, interfere with biological processes. Examples include coal dust, asbestos fibers or finely divided silicon dioxide, all of which can ultimately be fatal if inhaled. Corrosive chemicals possess physical toxicity because they destroy tissues, but they're not directly poisonous unless they interfere directly with biological activity. Water can act as a physical toxicant if taken in extremely high doses because the concentration of vital ions decreases dramatically if there's too much water in the body. Asphyxiant gases can be considered physical toxicants because they act by displacing oxygen in the environment but they are inert, not chemically toxic gases.
- As already mentioned, radiation can have a toxic effect on organisms.[3]
I understand that the two excerpts above are lengthy and might be redundant. Although, when the toxicity of an ingredient is taken into account, the mechanism by which the chemical or agent takes in damaging organs is crucial toward understanding the overall toxicity. In the Jergen's Skin Firming Moisturizing Lotion, there were around 29 ingredients. To break down the toxicity of each compound would be cumbersome. The four elements of 'Risk-Assessment' are: Hazard Identification, Dose-Response Assessment, Exposure Assessment, and Risk Characterization.
The terms listed at the end of the last paragraph might sound dangerous. The manufacturers are responsible for listing the information regarding the four elements listed above. You might be wondering the following:
Where do I find toxicity information?
If you are a chemist, the first place you might look is at the "Materials Safety Data Sheet". Right about now, you are probably wondering the following question:
How do I find the 'msds' sheet for a given chemical?
The process is easy. Type into a search engine like "Google" the following: Propyl Paraben msds . The result is shown below for the 'msds' for Propyl Paraben:
If you click on the 3rd option that appears to be a 'pdf' then the entire (5 page) 'Material Safety Data Sheet' will be downloaded. You will notice that there are 16 sections boxed out on the form as follows:
1) Chemical Product and Company Identification
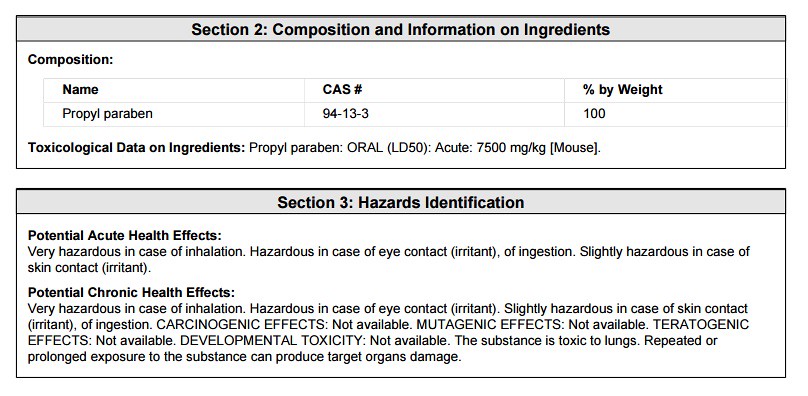
2) Composition and Information on Ingredients
3) Hazards Identification
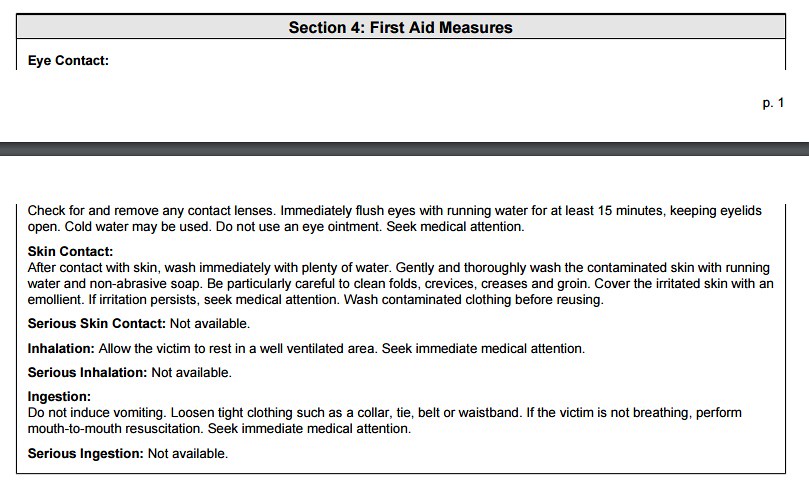
4) First Aid Measures
5) Fire and Explosion Data
6) Accidental Release Measures
7) Handling and Storage
8) Exposure Controls/Personal Protection
9) Physical and Chemical Properties
10) Stability and Reactivity Data
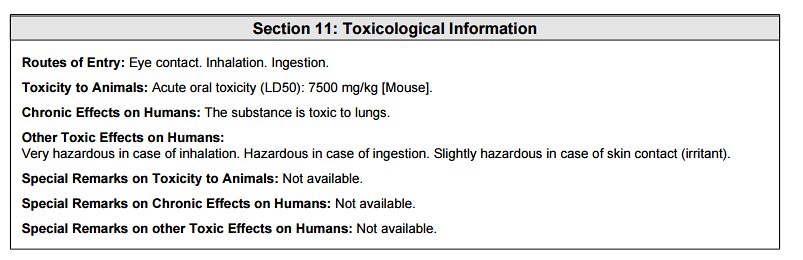
11) Toxicological Information
12) Ecological Information
13) Disposal Considerations
14) Transport Information
15) Other Regulatory Information
16) Other Information
Is that list comprehensive enough for you?
The purpose of the 'msds' information is to give the chemist or any other person working with a chemical the 'heads up' in terms of the properties and hazards associated with the chemical. In this case, the chemical is Propyl Paraben -- which is a preservative in Jergen's Skin Firming Moisturizing Lotion.
In terms of toxicity of a given chemical, a toxicologist will look the four elements listed above. From the 'msds' information, the most important factor will be the "LD50" value. According to 'Wikipedia' the 'LD50' value is defined as:
In toxicology, the median lethal dose, LD50 (abbreviation for "lethal dose, 50%"), LC50 (lethal concentration, 50%) or LCt50 is a measure of the lethal dose of a toxin, radiation, or pathogen. The value of LD50 for a substance is the dose required to kill half the members of a tested population after a specified test duration. LD50 figures are frequently used as a general indicator of a substance's acute toxicity. A lower LD50 is indicative of increased toxicity.
With units of dose/body mass:
The LD50 is usually expressed as the mass of substance administered per unit mass of test subject, typically as milligrams of substance per kilogram of body mass, sometimes also stated as nanograms (suitable for botulinum), micrograms, or grams (suitable for paracetamol) per kilogram. Stating it this way allows the relative toxicity of different substances to be compared, and normalizes for the variation in the size of the animals exposed (although toxicity does not always scale simply with body mass).
The above definition is worded in a complicated fashion. But what if you are interested in the general data surrounding the ingredient. Is the ingredient toxic in cosmetic products? If you choose to look at the 'msds' for the ingredient Propyl Paraben, you will find the following sections useful as shown below taken as screenshots of the downloaded 'pdf':
The first section shown above is the general information regarding the chemical Propyl Paraben. Note that the information contained on the following parts of the 'msds' apply to the chemical's use in a variety of manufacturing situations. Which is why the 'msds' sheet is not printed on the back or included with a cosmetic product. The next section is useful (probably the most useful) to the consumer of a cosmetic product -- "Composition and Information on Ingredients":
Upon first inspection of the ingredient information, you are probably wondering why this ingredient would ever be used in a cosmetic or food product. The above information exemplifies why the parameter of "concentration" is so important. In a typical cosmetic formulation, Propyl Paraben is probably around 5% of the total concentration. Therefore, the toxicity information of the LD50 is negligible. Below, I will show a calculation to prove such. The information above and below might be useful though to those with sensitive skin toward the class of chemicals known as "parabens". Below is the first aid measures as stated on the 'msds':
Again, the above information might be useful for consumers who develop a rash or adverse reaction toward this particular ingredient. The information contain in the section below pertains toward working with the chemical at a manufacturing level. Although, if you find an adverse reaction toward a product, you should report that product to the FDA.
The section above and below are rather redundant to the consumer, but necessary to the chemist working with the ingredient at the manufacturing scale level.
In the section above, the toxicological data was stated as being tested on a 'mouse model'. The stated LD50 was 7500 mg/kg of bodyweight. Which means that at a concentration of 7.5 grams per kilogram of bodyweight, the chemical would be toxic to an animal or person as shown below:
How would that lethal dose translate to a person who weighs 150 pounds?
How would a person figure that out given the toxicological data above?
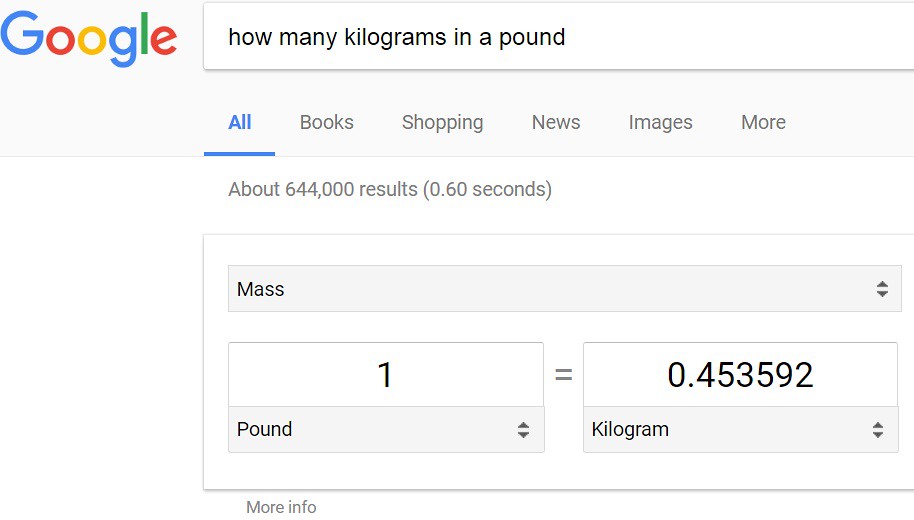
To start with, type into a search engine the following: how many kilograms in a pound? The answer below is given:
Next, the conversion of units from 'pound' to 'kilogram' is necessary as shown below:
With the proper units expressed uniformly across all values, the determination of the lethal dose is straightforward as shown below:
The result above indicates that in order for the chemical - Propyl Paraben to be lethal in the product - Jergen's Skin Firming Moisturizing lotion, the amount of Propyl Paraben contained in the bottle would have to be 510 grams. How many pounds is that? See below:
The result indicates that the bottle of lotion would have to contain 1.12 pounds of Propyl Paraben to be lethal to a human being. Obviously the bottle of lotion only has a total weight of 16.8 fluid ounces which is equal to 1.06 pounds. Meaning the entire bottle would have to be Propyl Paraben to be lethal to your body!!!!!
That was a useful exercise for the novice chemist.
What about the average consumer who does not want to mine through the confusing paperwork above -- the 'Materials Safety Data Sheet'?
Where else can a consumer find the equivalent information shown above stated more clearly to the consumer?
In the next section, a website will be given where you the consumer can easily find the equivalent information above stated in a more simple manner.
Toxic Ingredients
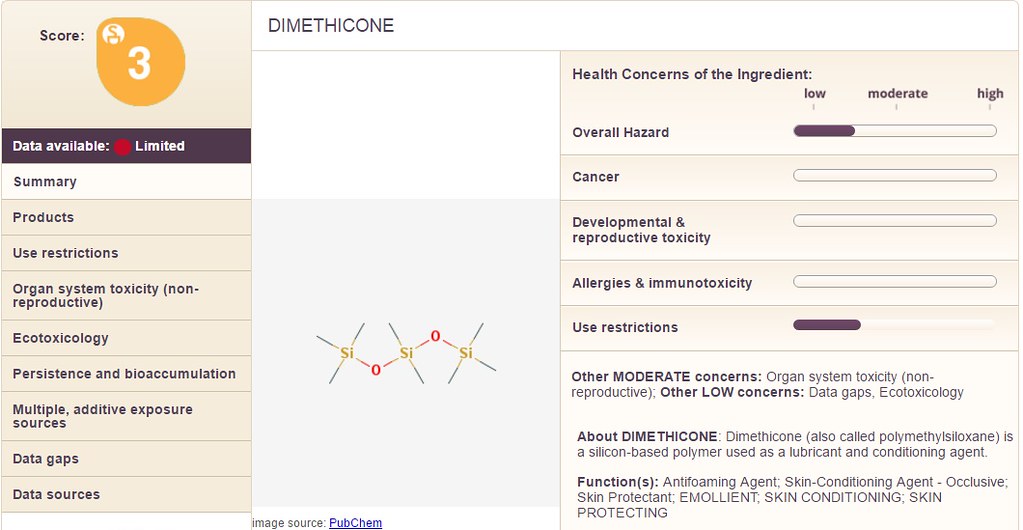
As a basic starting point, the site 'EWG' is great. The 'SkinDeep' database has some information. Take for example the ingredient 'Dimethicone'. Enter that ingredient into the database and look at the profile listed below:
Notice on the upper left hand corner, there is a 'score' which states the overall danger associated with the product (in this case Dimethicone). Inspecting the 'right hand column' there are the following categories:
1) Overall Hazard
2) Cancer
3) Development & Reproductive Toxicity
4) Allergies & Immunotoxicity
5) Use Restrictions
Note: Each of the above categories is measured along a scale with a range of "low" to "moderate" to "high".
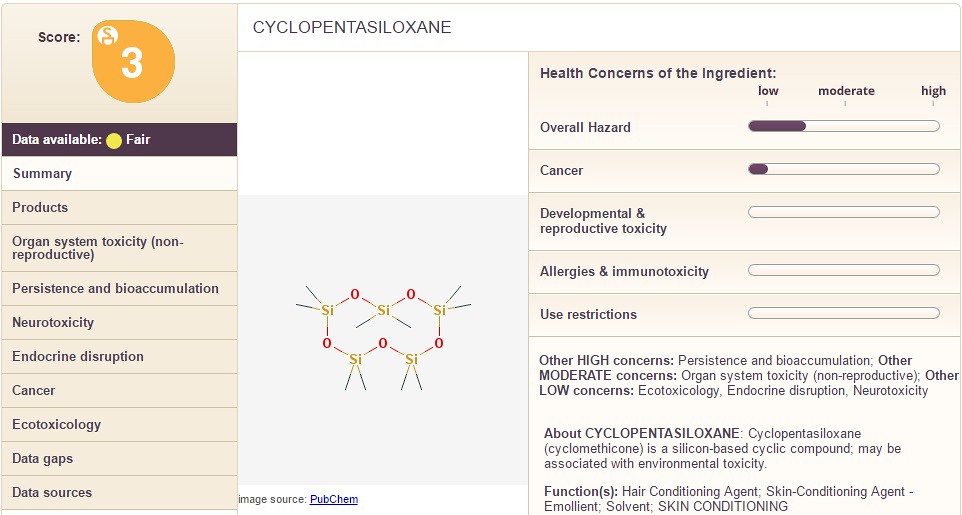
In the example of 'dimethicone' which is contained in the Jergen's lotion, the "overall hazard" is between "low" and "moderate". Additionally, the category "Use Restrictions" is also between "low" and "moderate". Whereas, the next chemical shown below is "cyclopentasiloxane" with a similar overall score of "3" but different values on the right hand side:
According to the sheet above, the chemical "cyclopentasiloxane" has a small amount of "cancer" risk. The scale is ambiguous and the 'msds' data would need to be consulted or the website 'EWG' for more information. Additionally, a consumer could choose to weigh the chemical above in comparison to another chemical like cetearyl alcohol as shown below:
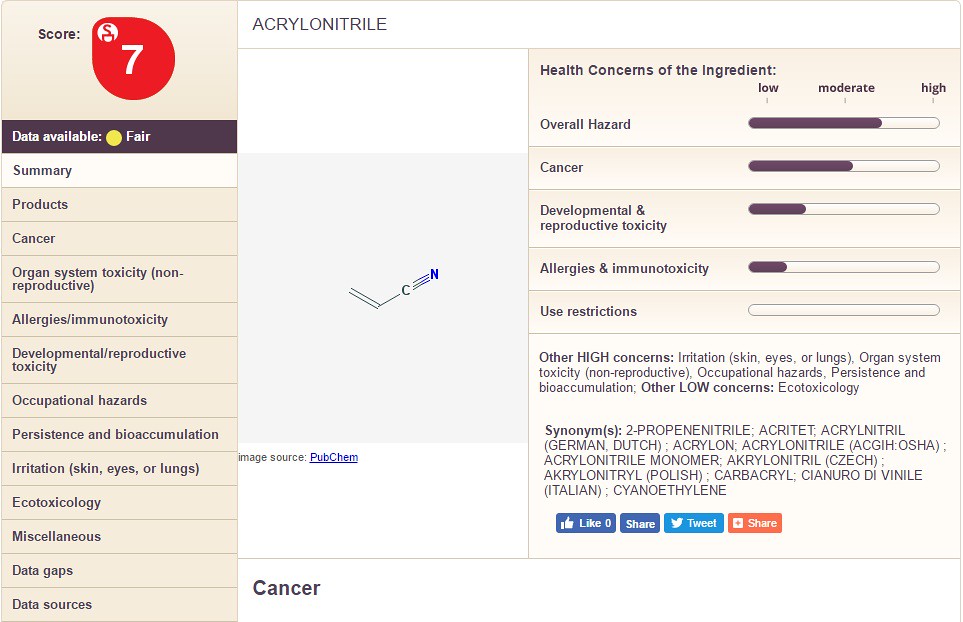
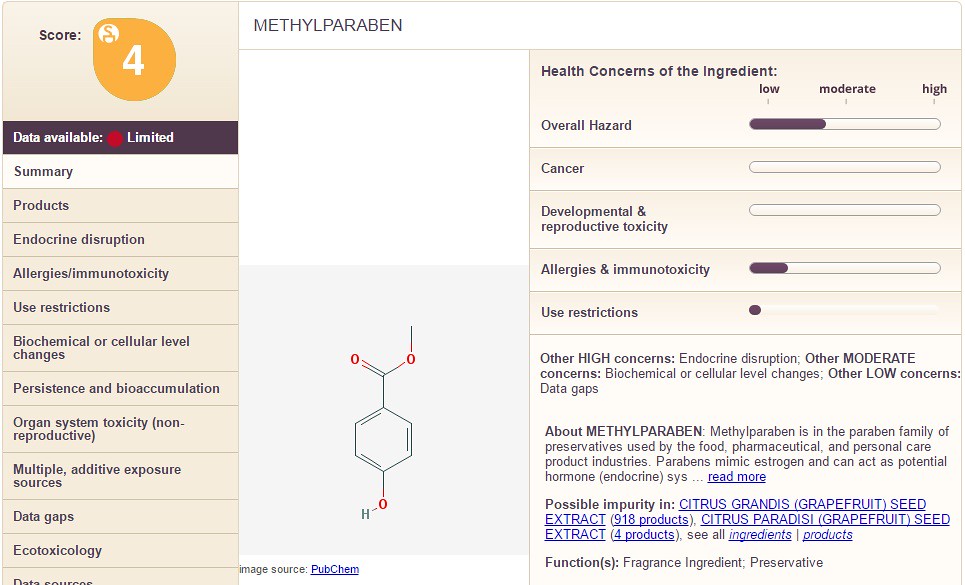
The only conclusion that can be drawn from the information is that cetearyl alcohol is less dangerous than "cyclopentasiloxane". Below I show a three more chemicals with different information for you to see: Acrylonitrile, Methyl Paraben, and Propyl Paraben. See if you can spot major differences between the three.
Methyl Paraben is shown below:
Propyl Paraben is shown below:
Notice how the chemicals differ in the categories on the right hand side along with the overall chemical score. In the images below, I take you through the columns on the left hand side (using Propyl Paraben as an example). The following sections filled with information are:
1) Products
2) Endocrine Disruption
3) Allergies/immunotoxicity
4) Use Restrictions
5) Developmental/Reproductive Toxicity
6) Ecotoxicity
7) Persistence/Bioaccumulation
8) Multiple, additive exposure sources
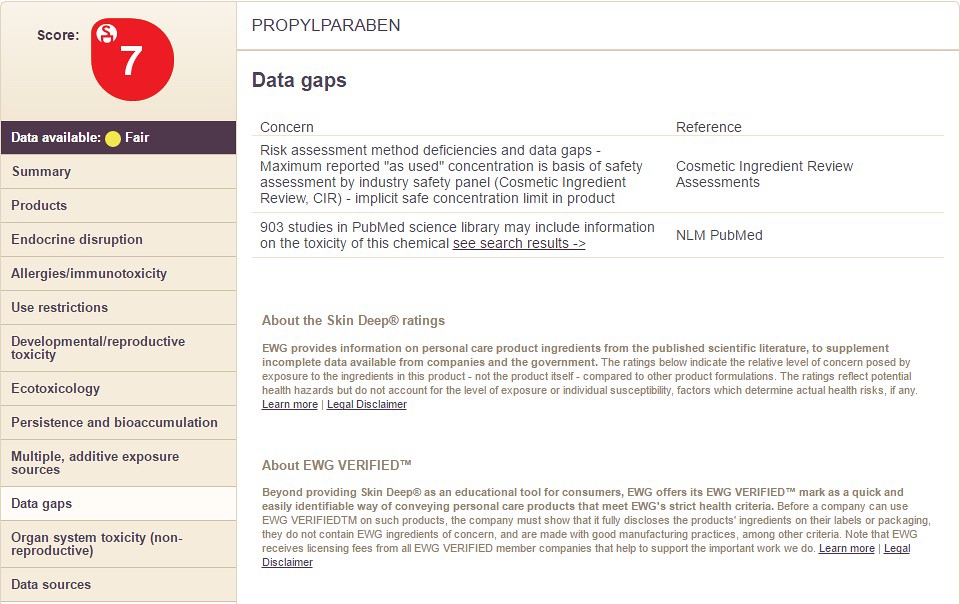
9) Data Gaps
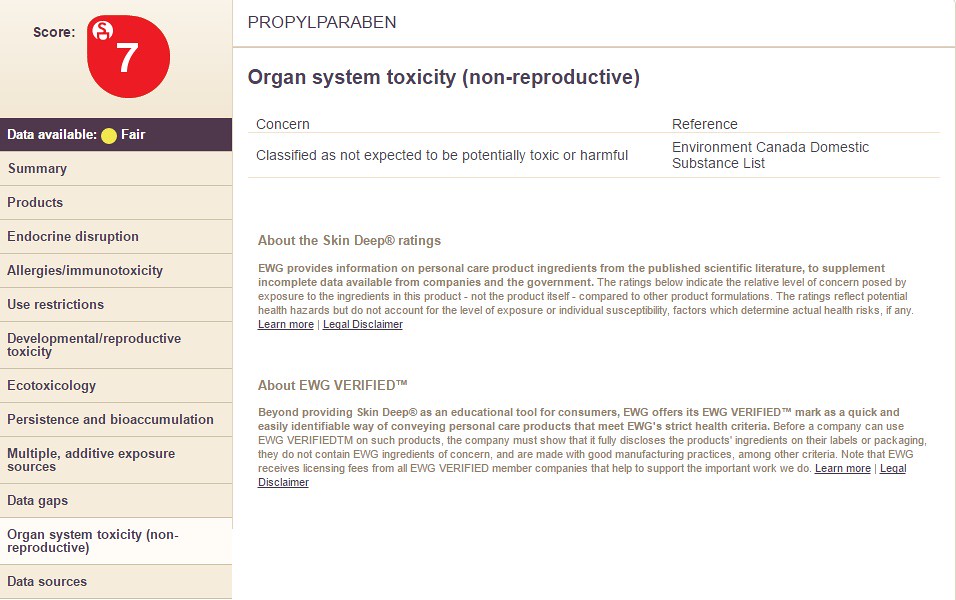
10) Organ System Toxicity
11) Data Sources
Upon clicking on the left hand column any of the categories listed above, a detailed page appears. For instance, click on the category for "products" and a list of over 3600 products will appear with the chemical Propyl Paraben in them as shown below:
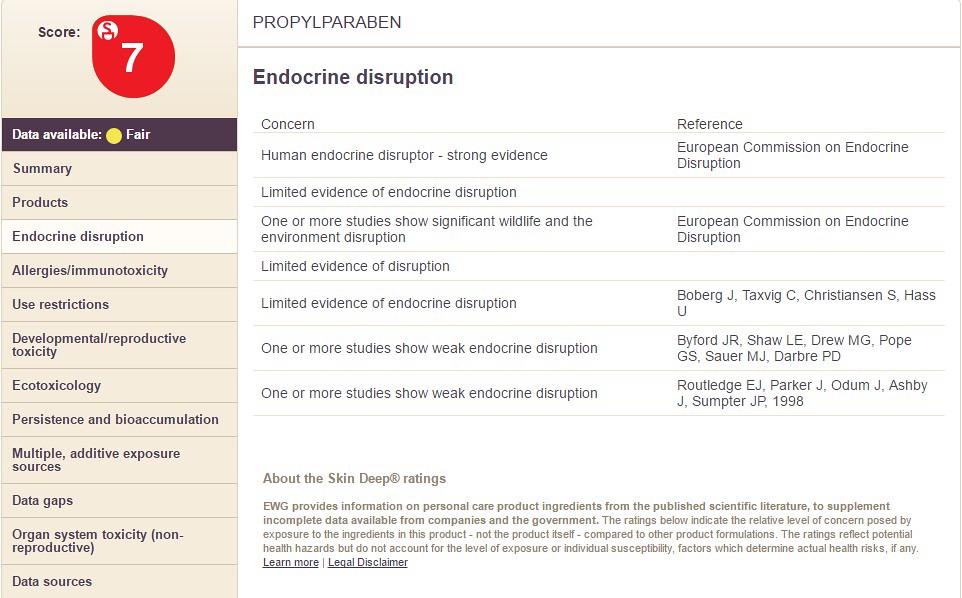
For "Endocrine Disruption" information, click and see the following below:
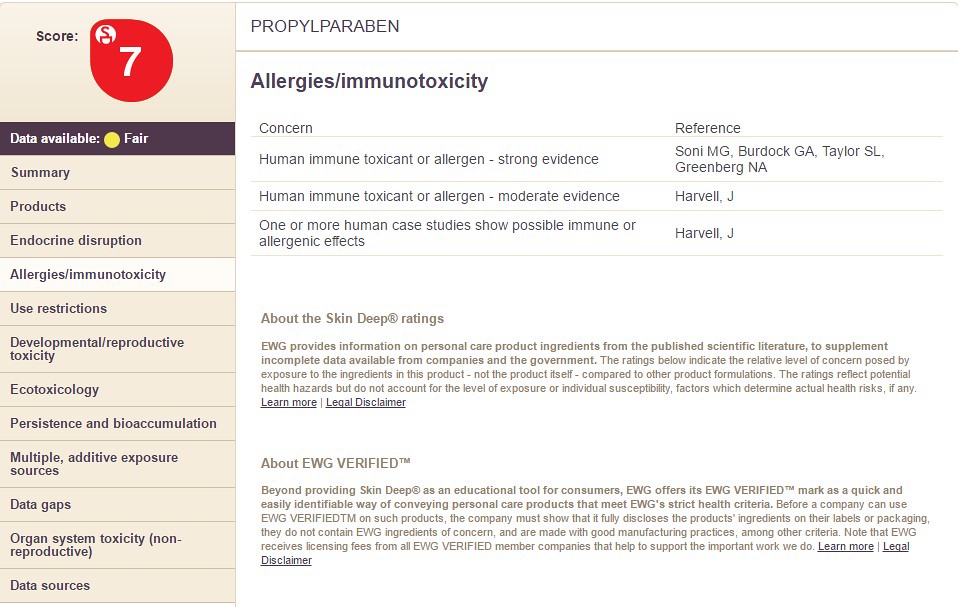
Choosing the option "Allergies/Immunotoxicity" will display the following information:
Choosing the option "Use Restrictions" will display the following information:
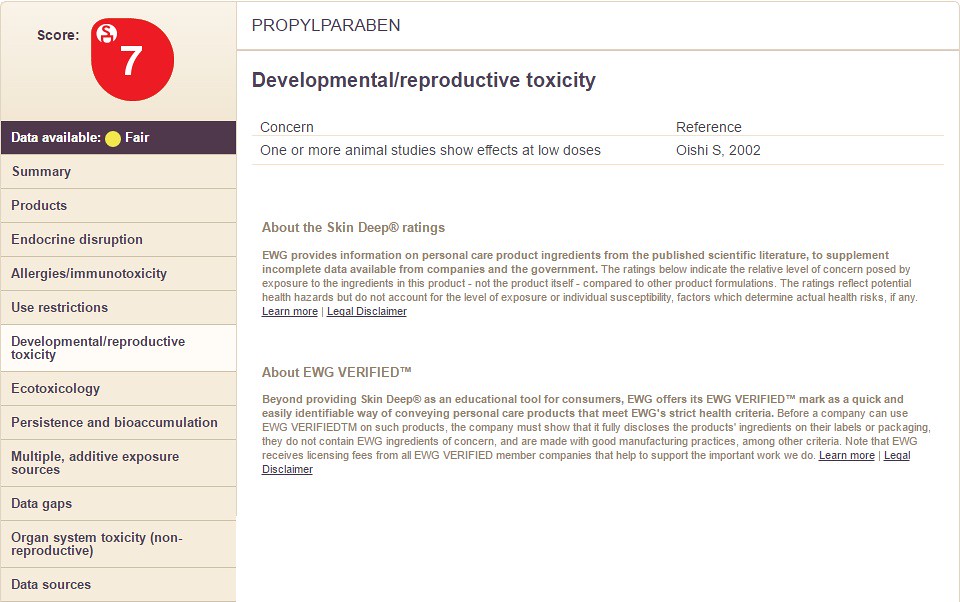
Choosing the option "Developmental/Reproductive Toxicity" will display the following information:
Choosing the option "Ecotoxicology" will display the following information:
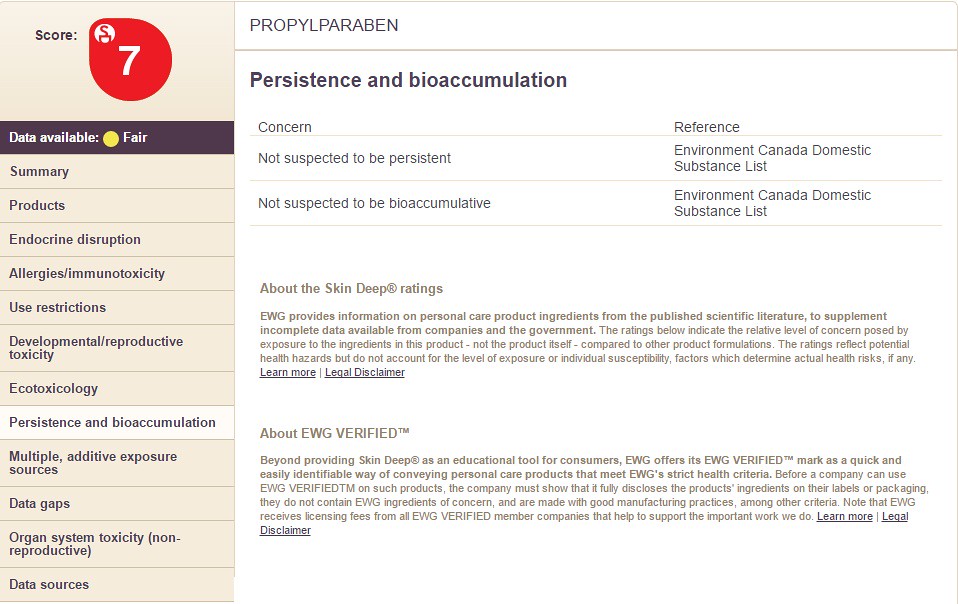
Choosing the option "Persistence and Bioaccumulation" will display the following information:
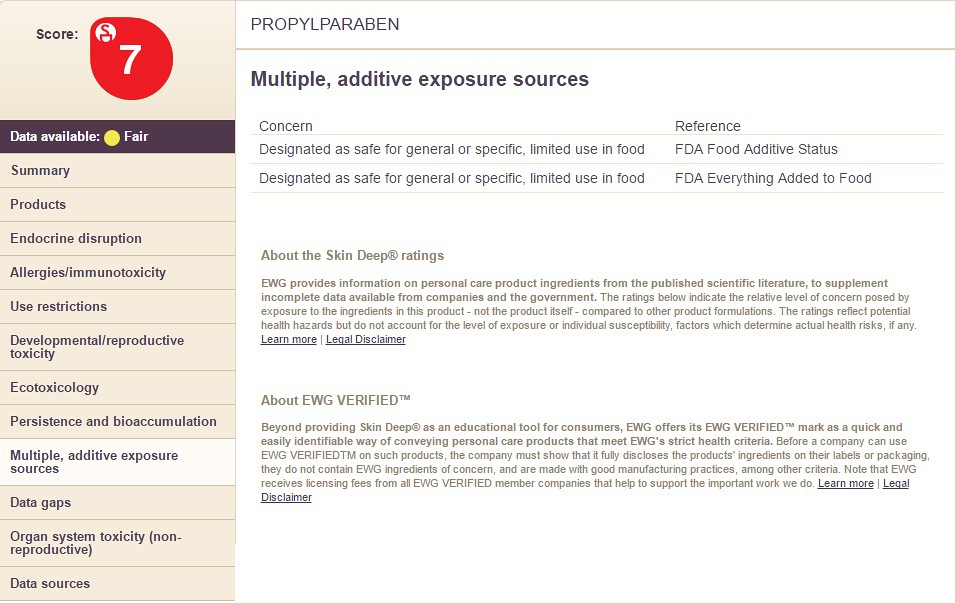
Choosing the option "Multiple, Additive Exposure Sources" will display the following information:
Choosing the option "Data Gaps" will display the following information:
Choosing the option "Organ System Toxicity" will display the following information:
As you can see from the above information, the amount of toxicity data available on the internet regarding a chemical ingredient is quite extensive and might be overwhelming at first sight. Keep in mind though that there are other parameters that need to be considered when viewing any of the sections listed above.
For instance, the chemical Propyl Paraben is considered toxic and should not be inhaled according to the "Materials Safety Data Sheet" -- then why would that ingredient be in a cosmetic product. Well, as part of a skin lotion formulation, the concentration (at 5% or less) is very low toxicity to begin with. Additionally, the matrix (formulation) in which the ingredient is in will not allow the chemical to diffuse into your skin very easily or be breathed nonetheless.
Conclusion...
The ingredients listed on the first part of the series for Jergen's Skin Firming Moisturizing Lotion all carry a degree of toxicity associated with them. In the examples shown above, some of the chemicals like cetearyl alcohol have a lower "overall hazard" associated with them compared to the "overall hazard" associated with Propyl Paraben.
Although, if you find yourself concerned, the information is available to greater investigate the chemical properties and toxicities associated with them as I have shown above. I provided a few examples from the ingredient list on the backside of Jergen's Skin Firming Moisturizing Lotion. Now, you can search the "EWG" database for the toxicity of the remaining ingredients. Additionally, I provided only one example of a site with the toxicity data displayed. Upon further inspection, many more sources will be revealed.
In the next post on the "Anti-Aging Skin Series", we will explore a different product with claims of 'anti-aging' properties. After exploring a few products with claims surrounding 'anti-aging' you will begin to see 'common ingredients' which must be present to support claims.
Until next time, have a great day!



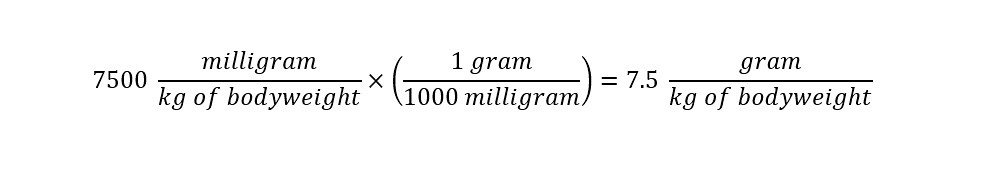
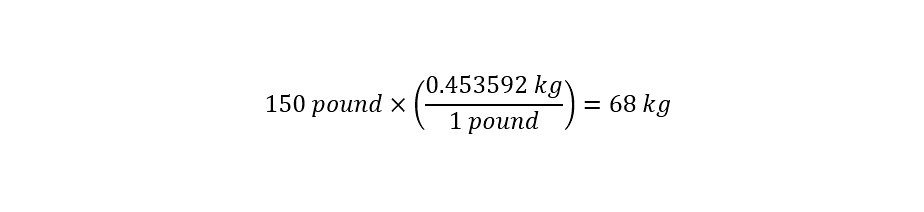
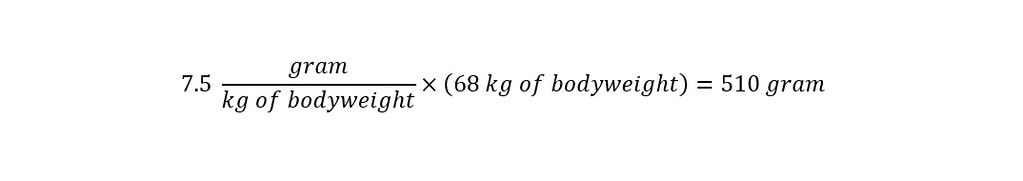






No comments:
Post a Comment