Source: Shutterstock
Where has that series gone called 'anti-aging skin care' products? For those new to the series, here is a quick refresher of the series. The point of the series was to demystify the world of cosmetics through discussing the ingredients on the back which make them up. How do ingredients help define the cosmetic product's function? I had to put the series on hold for a while (until now) do to other writing responsibilities. But now I am back. Thank you for being patient with me. I needed to learn how to engage you (the reader) more effectively. And give myself a little practice writing in other forums.
Anyways, I left off with analyzing a journal article about anti-inflammatory agents and skin barrier function. In the next blog post, I will continue where I left off. For now, I wanted to deviate a little and talk about skincare formulations and chemistry behind them. Specifically, I want to touch on 'emulsification.' Emulsifiers have an important role in skincare formulations. Read on below and find out how emulsifiers stabilize solutions of different phases. Enjoy!
A few months ago, I ran across an article (which is below) with a question which dives deep into the molecular interactions involved in an 'emulsifier'.
At this point right now, you may be scratching your head wondering what an 'emulsifier' is?
Fair enough.
If you think of the following bottle of Salad dressing shown below:
Source: Jesica Gavin
Notice how the Salad dressing on the left contains two distinct (i.e., very different) phases of liquids. The phase on top is 'less dense' (i.e. lighter, less matter per unit volume) than the phase on the bottom.
Phases with two different densities are around us each day. Ice water with ice cubes is the most frequent example. Ice is a phase of water that is less dense than liquid water. Meaning that there are fewer molecules per volume.
Water is a special substance.
Why? Typically, when the molecules in a liquid slow down to form a crystal, the unit density is larger than that of a liquid of the same substance. But I did not write this article to discuss one property exclusively -- i.e., density. If we dig a little deeper, the two distinct phases above can be made into a single phase. Of course, that would require a molecular phase with properties of both phases above.
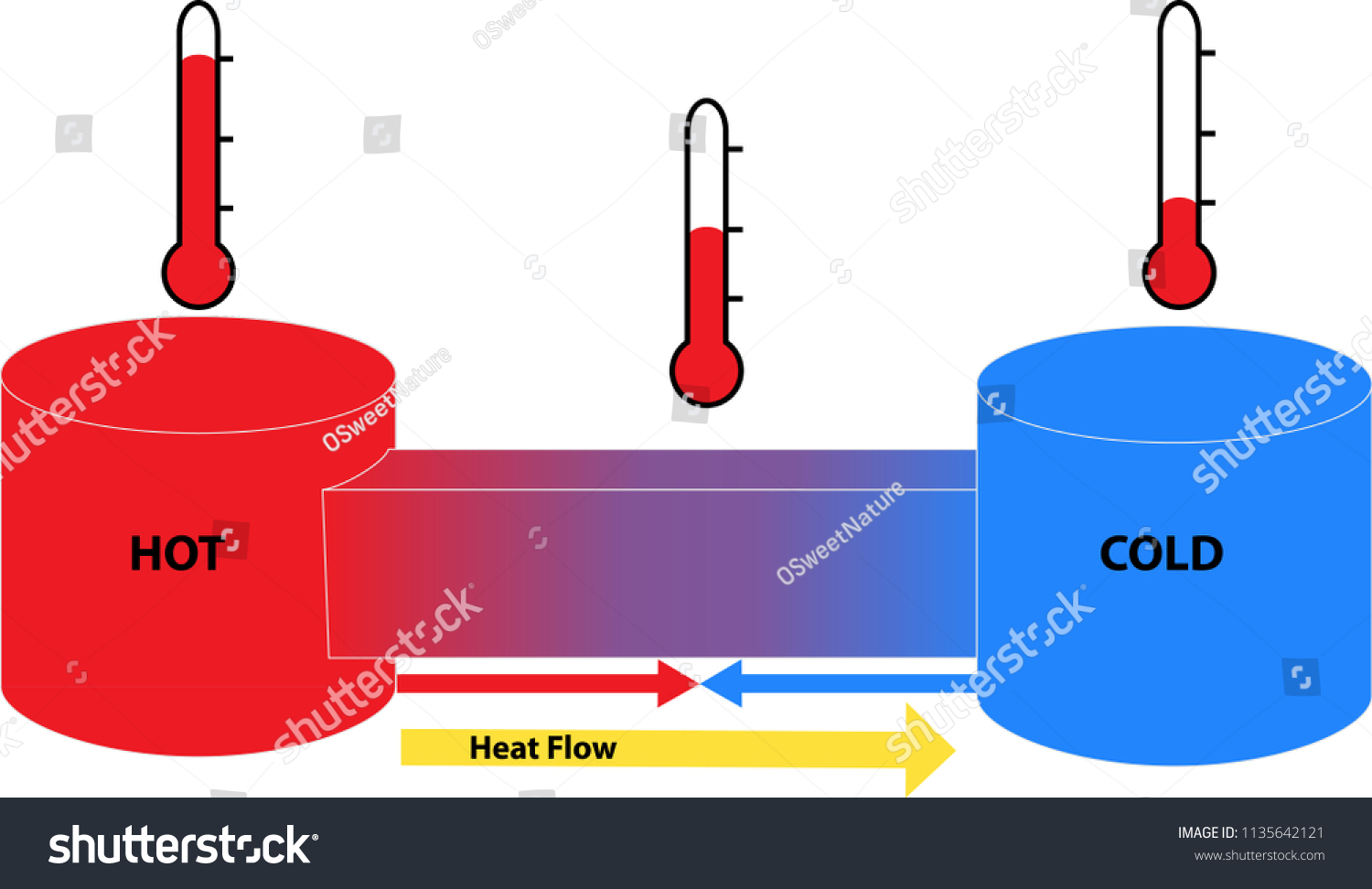
What is Thermodynamics?
Thermodynamics can be introduced by Wikipedia as follows:
Thermodynamics is the branch of physics that deals with heat and temperature, and their relation to energy, work, radiation, and properties of matter. The behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, chemical engineering and mechanical engineering, but also in fields as complex as meteorology.Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Nicolas Léonard Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars.[1] Scots-Irish physicist Lord Kelvin was the first to formulate a concise definition of thermodynamics in 1854[2] which stated, "Thermo-dynamics is the subject of the relation of heat to forces acting between contiguous parts of bodies, and the relation of heat to electrical agency."The initial application of thermodynamics to mechanical heat engines was extended early on to the study of chemical compounds and chemical reactions. Chemical thermodynamics studies the nature of the role of entropy in the process of chemical reactions and has provided the bulk of expansion and knowledge of the field.[3][4][5][6][7][8][9][10][11] Other formulations of thermodynamics emerged. Statistical thermodynamics, or statistical mechanics, concerns itself with statistical predictions of the collective motion of particles from their microscopic behavior. In 1909, Constantin Carathéodory presented a purely mathematical approach in an axiomatic formulation, a description often referred to as geometrical thermodynamics.
The introduction above may seem confusing. Thermodynamics is concerned with the various forms of energy in a system and its surroundings. Some people find thermodynamics to be confusing and incomprehensible. Terms such as entropy and heat may throw a monkey wrench into a person's understanding of a chemical system. Although, thermodynamics is good at explaining various large scale properties of systems -- such as heat transfer, the entropy within the system, etc. Confused yet?
Anyways, during the learning process, I have scoured the internet for various explanations or clarifications to different concepts. That is part of an investigation into learning about cosmetic products. Incorporating the chemistry behind the product requires a person to think about the energy within a system. How is the system work with its constituent ingredients? How does the system hold itself together? How does thermodynamics achieve this?
Thermodynamics is the accountant for the energy of the system. A person cannot help but investigate thermodynamic properties of a system to understand how the individual molecules mixed into a solution interact such that collectively they form the macroscopic system -- i.e., cosmetic product.
One such concept was that of an "Emulsifying Stabilizer". Below is an example of a question/answer query that I found on a website called "Research Gate":
Question:
How does an emulsifier stabilize an emulsion?Emulsion can be stabilized by increasing the repulsion between the dispersed phase i.e., by increasing the electrostatic repulsion (which is long range) or steric repulsion (short range). Emulsifiers are amphiphiles that reduce the interfacial tension between the two phases and contribute to the stabilization of dispersed droplets with electrostatic or steric effects.I wish to know the detailed mechanism by which emulsifiers stabilize an emulsion. References will be of real help.Thanks.
One possible Answer that intrigued me was the following:
Answer:
When a surfactant adsorbs on the interface the interfacial tension between the two phases decreases. The reduced interfacial tension depends on the concentration of the surfactant according to the Gibbs’ isotherm.Adsorbed surfactants or solid particles stabilize emulsions via two main mechanisms:1. steric stabilization2. electrostatic stabilizationSteric stabilization arises from a physical barrier to contact and coalescence. For example, high-molecular-weight polymers can adsorb on the surface of the dispersed phase droplets and extend significantly into the continuous phase, providing a volume restriction or a physical barrier for particle interactions. As polymer coated particles approach, the polymers are forced into close proximity and repulsive forces arise, keeping particles apart from each other. Surface-active solid particles such as clays have also been shown to sterically stabilize emulsions.Electrostatic stabilization is based on the mutual repulsive forces that are generated when electrical charged surfaces approach each other. In an electrostatically stabilized emulsion, an ionic or ionisable surfactant forms a charged layer at the interface. For an oil-in-water emulsion, this layer is neutralized by counter ions in the continuous phase. The charged surface and the counter ions are termed a double layer. If the counter ions are diffuse (thick double layer), the disperse phase droplets act as charged spheres as theyapproach each other. If the repulsive forces are strong enough, the droplets are repelled before they can make contact and coalesce, and the emulsion is stable.In general, electrostatic stabilization is significant only for oil-in-water emulsions since the electric double-layer thickness is much greater in water than in oil.Both electrostatic and steric forces can prevent aggregation or coalescence and hence stabilize emulsions.Reference: Urrutia P.I., Predicting Water-In-Oil Emulsion Coalescence From Surface Pressure Isotherms, Department of Chemical and Petroleum Engineering, University of Calgary M. Sc. Thesis, 2006.I hope it was useful for you.
That is what occurs when the mixture is shaken up as shown in the right-hand side. Over time, the emulsifier is not strong enough to stabilize the two phases from forming again. The interactions of repulsion and attraction play a dominant role in all of chemistry. The scale on which these interactions occur is so small that typically we do not see them (or think about them).
Chemists think about them on a daily basis -- especially, formulation chemists at a cosmetic product manufacturing company. The energetics associated with interactions determine whether a reaction will occur or not. Chemistry plays a beautiful role in our world every day. Although, most of us choose not to look or think of these interactions. Which is fine too.
Although, when a consumer goes into a store and purchases a cosmetic product, the questions he/she asks the vendor are involving the interactions of the ingredients. Further, the questions include the interaction of the ingredients with the customer's skin and thermodynamic variables -- temperature, pressure, volume, and environmental conditions. After reading this brief article, I would expect your picture and thinking involving cosmetic ingredients to have widened -- which is great.