There was a scene where two surgeons were talking small talk in the doctor's lounge on the show. Who they were is not necessarily important. At one point in the conversation, one holds up a bottle of lotion and says, "We are doctors and we don't even know what the chemicals are listed on the back of this lotion....What is dimethicone?" At that point, I perked up and thought of the blog that I was in the process of writing for the "Anti-Aging Skin Series" which originated out of the long article that Kayla asked me to simplify titled "Can Science Really Reverse Aging Skin?". The chemical dimethicone is an ingredient that was discussed in a previous blog post. I decided to scrap the first draft that I had and start over. We last left off with a glossary of terms which would help us in understanding the ingredient list on the backside of a bottle of lotion -- Jergen's Skin Firming Moisturizing Lotion (previous blog).
The above introduction proves that an interest does exist for an understanding of the chemicals which are listed on the back side of products. Even if the interest was stated on a Hollywood set in "Grey's Anatomy". In the next blog post, I will tell you a real story about a chemist with a similar medical experience.
With this in mind, lets explore the backside of the ingredients which were listed in the previous blog post for the moisturizing lotion made by Jergen's -- which supposedly leaves a younger and healthier looking skin after use. A picture of the front side of the bottle is shown below:
Ingredients
To start out with, I must admit that I have had difficulty in trying to decide the format of these blog posts with the ingredients listed on the back of the container and their associated toxicities. In light of this revelation, bear with me through the next few blog posts. I am finding my way in terms of design and information layout. My plan is to lay out the ingredients in this blog with the chemical structures shown along with a brief discussion of the grouping/function of them relative to the product description. In the next blog post, I will discuss briefly a few aspects of the associated toxicology of the ingredients. The learning curve was much longer (took me way longer than I had imagined -- a month and a half) to find my way. But we are here now and lets get started.
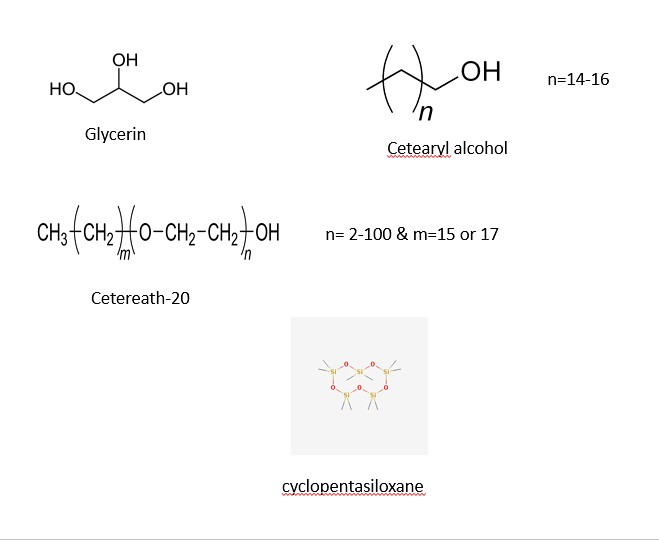
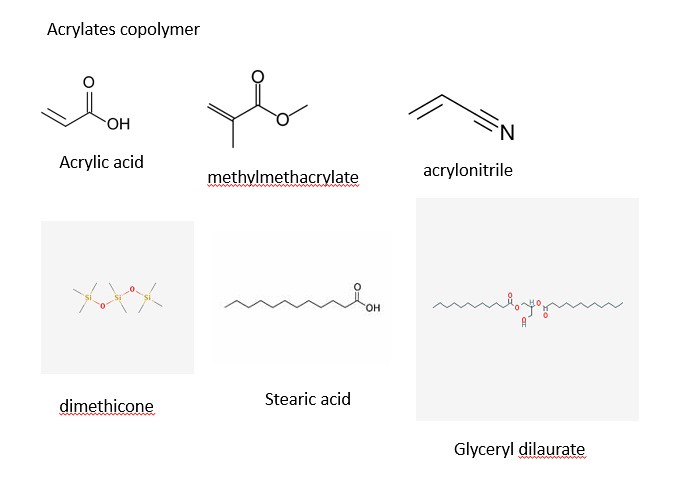
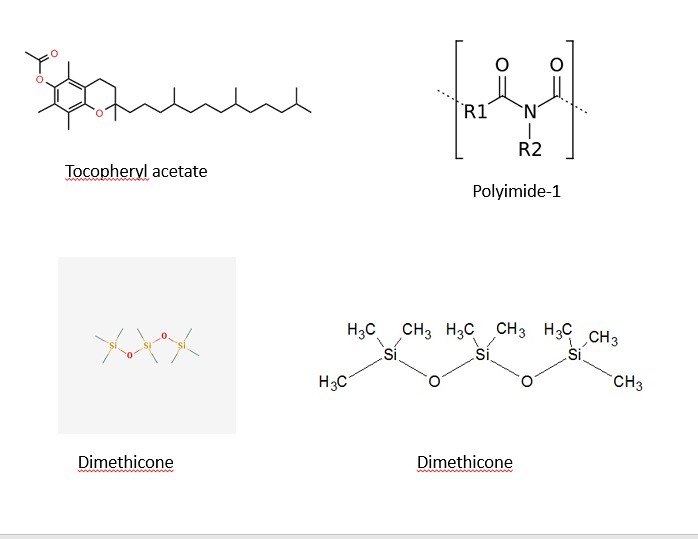
The structures of most of the ingredients are shown in the three panels below. Initially, I decided to take structures from two main websites: Wikipedia and the Environmental Working Group. After some time, I decided to learn a program (that I have no used in quite a while) to draw the chemical structures myself. The advantage of drawing the images myself with a chemical sketch program is that I am allowed to orient the molecules my own way to illustrate the point of function. With that being said, the four images below contain the chemical structures listed in the ingredients section on the back side of a bottle of Jergen's Skin Firming Moisturizer Lotion as shown below:
The three panels above show a variety of chemical structures. Some of which might be recognizable and others that are not. Note: the structures above were taken from different sources and might appear different. I will discuss that shortly for clarification purposes. I will be discussing the function of the ingredients in the product and talking periodically about the chemical structure, so feel free to scroll the mouse back up to the picture to view the structures as we move along.
These are some of the ingredients listed on the back of the bottle of lotion as shown below:
To start with, figuring out the function of the ingredient is relatively easy. In the previous post, I listed the function and definition of each ingredient. The important connection is to make between the function and the reason why the manufacturer chose to incorporate the ingredient into the lotion.
Two questions can be offered at the start in order to understand the ingredients:
1) Why is the ingredient in the product?
2) What is the concentration of the ingredient in the bottle?
These questions might seem redundant, but in actuality, chemists ask themselves these two questions before starting the research and development phase. Further, during the testing phase, these questions come up arise again if the product does not meet the intended purpose. For instance, does the cream spread smoothly over the surface of your skin without 'caking up'? Part of the answer lies in understanding the concept of "hydrophilic" and "hydrophobic". The concept of "hydrophobic" and "hydrophilic" is sometimes difficult to convey. Here are two videos taken from YouTube that are each less than 5 minutes in length and might help clarify the distinction between the two terms.
Source: Science Friday
As you can see, the two components have separated completely. If you were to shake the bottle up, the two layers would mix for a short (a few minutes) duration until the two layers. Here is a short explanation of how salad dressing works from our website. There are numerous explanations out online to clarify the distinction. One explanation that I found helpful was from an episode of the wildly popular show called "Science Friday". The episode was called "Salad Dressing Science." Here is a very simple explanation:
Most vinegars are solutions of acetic acid and water (plus some other acids and alcohols, depending on the type of vinegar you are using). Water, acetic acid, and alcohol are all examples of polar molecules—molecules that have a slightly negative charge at one end, or pole, and a slightly positive charge at another end. These slightly charged poles arise because one or more atoms in the molecule are electronegative, meaning that they tug electrons—which are negatively charged—towards them, creating an uneven distribution of charge within the molecule. Polar molecules are generally attracted to other polar molecules because their slightly negative poles have an affinity for their slightly positive poles. Polar molecules are attracted to water molecules—which are also polar—and are called hydrophilic, which means “water loving.”Oils are a different story. Oils are a type of fat (like butter, shortening, and lard) and are considered non-polar. Fats and oils are composed primarily of long molecules called fatty acids (usually bound together by glycerol molecules into groups of three called triglycerides). Most of the atoms in a fatty acid molecule share electrons evenly and are neither negatively nor positively charged (although fatty acids do contain small regions of polarity—just not enough to make the whole molecule polar.) Non-polar molecules love other non-polar molecules and will glom together when mixed with water. You can observe this phenomenon by placing a few drops of oil on the surface of a bowl of water—eventually the drops will form a single large oil slick. Oils repel polar molecules such as those found in vinegar. Because oils also repel water, they are called hydrophobic, which means “water-fearing.”
The natural question arises when considering how the two layers stay together for a short time:
What holds the two layers together?
Obviously, whatever chemical that holds the two layers together will have to contain both properties. That is a long chain molecule could serve as the linker. Although, the linker (or emulsifier) molecule has to have a hydrophobic end along with a hydrophilic end too.
What does a molecule that serves as an "emulsifier" look like?
Here is a photo of the linker molecule shown on the webpage below:
Source: Science Friday
Notice how the molecule in the picture has both a polar and a non-polar end. Again, from the website "Science Friday" for the episode "Salad Dressing Science" -- a short excerpt explaining the function of an "emulsifier":
How can we bring together polar and non-polar molecules to make something delicious like mayonnaise (which is essentially a combination of water and oil) or salad dressing? We need an emulsifier. Emulsifiers are the hand-holders of the molecule world. They contain both hydrophobic and hydrophilic regions and are able to attract and “hold hands” with polar and non-polar molecules simultaneously, pulling them together to form a special type of mixture called an emulsion. For instance, after adding an effective emulsifier to oil and vinegar and mixing thoroughly, separation of the oil from the vinegar will take much longer or won’t happen at all.
At this point, you are probably thinking the following question:
What does salad dressing science have to do with producing a cosmetic formulation?
Actually, from a chemist's standpoint, there is no difference. Both are mixtures of chemicals. Furthermore, each chemical has a distinct purpose (or supportive purpose) in the product formulation. Lets start with the categories that each chemical is partitioned into. In certain instances, some chemicals will play a dual role. This will become clear shortly in the next section.
Categories Of Chemicals
In the previous post which contained a dictionary of 'functions' that chemicals play in cosmetics and food chemistry. Before that, the previous post was the first post in which I chose a product as shown above -- Jergen's Skin Firming Moisturizer lotion -- to break down. In that post, I listed each chemical with the function that each serve. Now, the time has come to group the chemicals into categories in their respective functions -- to drive home the function of the components.
A) Categories: Hydrophobic, Hydrophilic, Emulsifier, and Surfactant:
Above, there was a brief introduction into the 'hydrophobic'(water fearing) and 'hydrophilic'(water loving) chemicals. This distinction is very important since a large amount of chemicals are thought of in these terms in product formation. Additionally, there are 'linker' molecules which have both properties -- 'hydrophobic' and 'hydrophilic' -- which are extremely important to make a mixture appear to be uniform. This class of chemicals is known as 'emulsifiers'. I will refer to the previous post with the dictionary for more information.
Note: the categories listed below -- hydrophobic, hydrophilic, and emulsifier are used for simplifying purposes. Depending on the professional settings and synthesis, the degree of each categorical placement can be argued. I use these categories to help the reader understand that a certain amount of cosmetic formulations have a fatty component (nonpolar), a hydrophilic component (glycerin) and an emulsifier (cetearyl alcohol) to make a mixture. Other chemicals are present to assist in stabilizing the mixture to hold together along with other attributes (properties) which will be highlighted below.
A.1: Hydrophobic Ingredients
The first category will be the 'hydrophobic' compounds (i.e., non-polar, oils, fats, starch, etc.) shown below:
1) Mineral Oil
2) Petrolatum
3) Cyclopentasiloxane
4) Acrylates Copolymer
5) Dimethicone
6) Stearic Acid
7) Glyceryl Dilaurate
8) Fragrance
9) Methyl Paraben
10) Butylene Glycol
11) Acrylates/C10-C30 Alkylacrylate Crosspolymer
12) Propyl Paraben
13) Hydrolyzed Wheat Protein/PNP Crosspolymer
14) Tocopheryl Acetate
15) Centella Asiatica Extract
16) Cocos Nucifer (coconut oil)
17) Hydrolyzed Collagen
18) Withania Somnifera Root Extract
19) Fusus Vesiculosus Extract
20) Hydrolyzed Elastin
Upon inspecting the ingredients in the list above, you will notice that some of them do not have chemical structures shown in the image panels above. Which raises the following question:
Why are their chemical structures not shown?
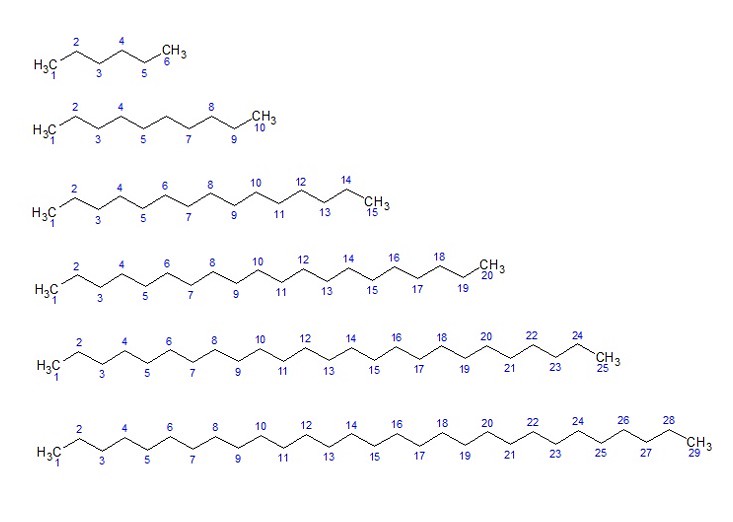
The reason is that oils contain mixtures of fats along with other long chain carbon molecules. The chains are nonpolar and are repeating carbon atoms with hydrogen atoms attached in most cases. In the image below, I show a distribution of carbon chains of varying length to illustrate my point:
An example of such a mixture of carbon chains is Petrolatum. Petrolatum is a jelly with viscous properties. The viscosity can be controlled by the distribution of chain lengths in the mixture. For a mixture with a high viscosity, the chain length might be greater than 25 carbons in length. Whereas, for a less viscous mixture, the carbon chains might be in the range of less than 25 carbons in length.
In the image above, I show carbon chains
A.2 Hydrophilic Ingredients
The next category is the opposite of the first in properties -- the 'hydrophilic' compounds (i.e., polar, ionic molecules, water, short chained alcohols, etc.) are shown below:
1) Water
2) Glycerin
3) DMDM Hydantoin
4) Sodium Hydroxide
5) Arginine
6) Polyimide-1
A.3 Emulsifier Ingredients
Finally, last but not least, the category of the 'linker' molecules -- 'emulsifier' compounds (i.e. surfactants, soaps, detergents, etc. ) are shown below:
1) Cetearyl Acohol
2) Acrylates/C10-C30 Alkylacrylate Crosspolymer
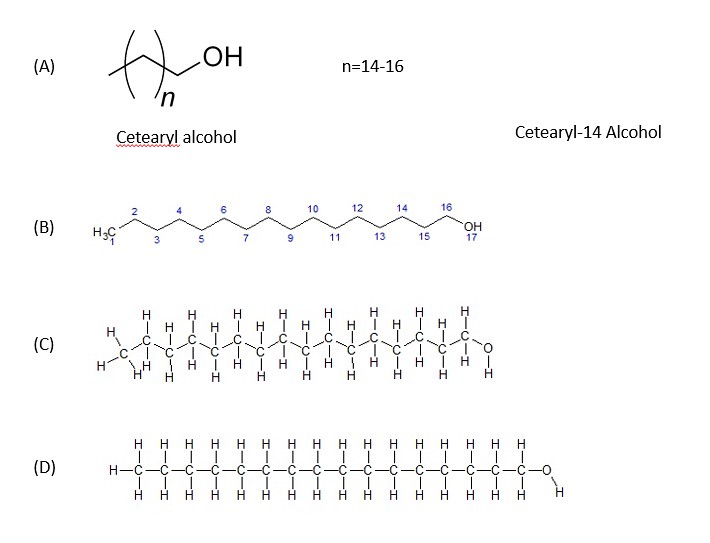
In this mixture, there are few emulsifiers present. I must note that the structure for cetearyl alcohol is rather ambiguous in the picture above. You probably do not understand what chemical structures with parenthesis () mean? In the image below, the chemical structure is expanded to the chemical cetearyl alcohol-14:
Shown above are 4 different illustrations of cetearyl alcohol. Since the length of the alkyl (carbon chain) can vary based on the index (the number outside of the parenthesis), cetearyl alcohol has many different structures. Meaning that the length of the carbon chain will vary. As you can probably guess, the longer the carbon chain, the more that the molecule will acts as a 'nonpolar' one rather than a polar molecule. short chained cetearyl alcohols are 'polar'.
In the structure (B) above, cetearyl alcohol-14 chemical structure is shown without the hydrogen molecules attached. This representation is referred to as the 'line structure.' Often chemists will use the 'line structure' to simplify the representation to focus on other aspects of the molecular structure. Structure (C) has all of the hydrogen atoms attached to the structure. Structure (D) shows the full structure flattened out for clarity of the numbers and types of atoms present. In future blogs, I might just use line formulas to simplify the case in point of ingredients in a given product formulation -- be aware!
A.4 Surfactants
Note: these are not the only three categories of ingredients. There are other main categories which are slightly separate from the above three categories. One such category is surfactants -- which are listed below:
1) Ceteareth-20
2) Aluminum Starch Octenyl Succinate
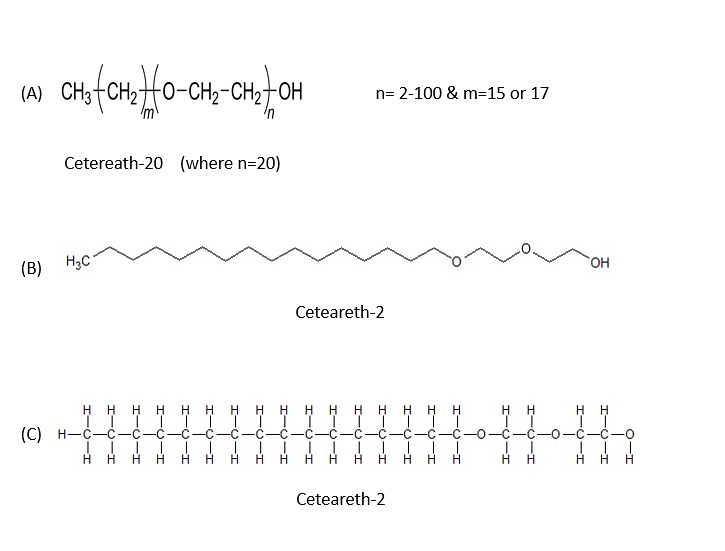
Here is the structure of ceteareth-2 shown in the image below:
Again, shown above are 4 different structures for ceteareth-2. Notice in the structure (A) that in parenthesis there is one "O" on the chain? If the index 'n' is two, there will be two as shown in the line structure (B). If the index 'n' was 20 as in ceteareth-20, then there would be 20 "O" (oxygen atoms) and the molecule would run off of the page of the screen. Therefore, I used an example of ceteareth-2 to show the chemical structure.
B) Additional Categories: Skin Conditioning Agent, Skin Protectant, Humectant, and Emollient:
B.1 Skin Conditioning Agent
1) Glycerin
2) Mineral Oil
3) Cyclopentasiloxane
4) Dimethicone
5) Glyceryl Dilaurate
6) Butylene Glycol
7) Arginine
8) Tocopheryl Acetate
9) Centella Asiatica Extract
10) Hydrolyzed Collagen
11) Withania Somnifera Root Extract
12) Fucus Vesiculosus Extract
13) Hydrolyzed Elastin
Inspecting the list above and comparing it to the three additional lists below, some chemicals will serve two purposes. This multifaceted nature of the chemical gives rise to the distinction of definitions of these categories. Meaning that a skin-conditioning agent is also an humectant and an emollient in certain products. The important take-away message is that in order to interpret the function of various ingredients, look at the product. Ask yourself the following question:
What is the purpose of adding the ingredient into the product?
To determine the function, the website EWG gives the categorical function (i.e., skin-conditioning agent, skin protectant, humectant, etc.). In this blog post, the product is a 'skin firming moisturizing lotion' from the manufacturer Jergen's. The list above gives a few ingredients that will not be common across various lotions (products) from different manufacturers. Alternatively, the majority of the chemicals are common ingredients. This will become apparent in the future posts which look at different products.
Skin conditioning agent is defined in the previous blog as:
A skin conditioning agent and an essential fatty acid that helps maintain optimal skin health and function. Lipid Organic products found in living systems that are insoluble in water, like fats. Cell membranes are made of lipids.
The nonpolar nature of the chemicals will serve as a 'skin-repleneshing agent' to the lotion under inspection. As I mentioned in the very first post 'anti-aging' post is that there is very little in terms of regeneration of collagen by external chemical means possible. Therefore, the purpose of adding a distribution of lipids, oils, and fats will serve as a "coating" to adhere to the upper layer of the Stratum Corneum (the outer layer of the epidermis). If you were to add a highly polar liquid as the major ingredient to the lotion, the consistency would change and possibly not stick to the outer layer of the epidermis. Remember the following rule:
Like loves Like!
If you apply this principle in formulating products, then formulation will be much easier. As I mentioned, some ingredients serve many different purposes. Adding oils, fats, lipids that will adhere to the skin will produce a layer which might be perceived as 'new skin' or 'moisturized skin'. Really, the layer is just sitting on your skin. How do you ensure that the layer will stay on the skin?
In order to make that happen, you will need to add a binder and a skin protectant. Before moving to the next section of skin protectant, remember that skin conditioning agents will diffuse down into the top few layers of the skin -- but mostly sit on the outer layer.
One ingredient to highlight out of the skin-conditioning agent list is: Fucus Vesiculosus Extract. Research from 2002 suggests that the extract might have anti-aging properties as highlighted in the abstract to the research article shown below:
Recently the researchers found that an extract of Fucus vesiculosus, which is a type of seaweed, promotes the contraction of fibroblast-populated collagen gels through increased expression of integrin molecules. In this study, they investigated the effects of topical application of an aqueous extract of this alga on the thickness and the mechanical properties of human skin. A gel formulation that included 1% of the extract was applied topically to human cheek skin twice daily for five weeks. A significant decrease in skin thickness measured by B-mode ultrasound was elicited, as was a significant improvement in elasticity measured with a Cutometer as compared with controls. In cheek skin, the thickness normally increases and the elasticity usually decreases with age. These results suggest that the Fucus vesiculosus extract possesses anti-aging activities and should be useful for a variety of cosmetics.
The exact mechanism is not known. The research results were preliminary and more research has to be done to decipher into the exact mechanism of the Fucus Vesiculosus extract. This is a chemical that stands out. Other chemicals listed above will be redundant in other products. Next, I will comment on skin protectants below.
B.2 Skin Protectant
1) Glycerin
2) Petrolatum
3) Mineral Oil
4) Dimeticone
The list above are common ingredients which function as skin protectant. The purpose of adding a skin protectant is to ensure that the layer of lipids, fats, oils, long carbon chains to form a film on the top layer of the skin. Additionally, the purpose is to hold the moisture inside the upper most layer of the skin (to hydrate) which promotes the appearance of 'healthy and younger' looking skin. Using these ingredients does not actually change the physiology of the skin. Basically, the skin protectant serves as the outer layer of the skin -- i.e. fake layer.
Another purpose of having a skin protectant is to hold in the moisture in the top layer of the skin. The chemicals that promote moisture build up are 'humectants.' Humectants are chemicals which are 'hydroscopic' in nature. Hydroscopic means that the chemical will absorb water. Incorporating humectants into a moisturizing lotion serves two purposes: attracts water (keeps moisture in) and prevents the lotion from drying out (and cracking). Below is a list of humectants found in the Jergen's Skin Firming Lotion:
B.3 Humectant
1) Glycerin
2) Butylene Glycol
3) Hydrolyzed Collagen
B.4 Emollient
1) Cetearyl Alcohol
2) Petrolatum
3) Mineral Oil
4) Cyclopentasiloxane
5) Dimethicone
6) Glyceryl Dilaurate
7) Hydrolyzed Collagen
The list above contains the chemicals which act as Emollients. What is confusing to me is the vague distinction between the categories. This is the reason why I chose the distinction of 'hydrophobic' 'hydrophilic' and 'emulsifier'. These distinctions make more sense in formulating a cosmetic product. Here is the definition of an 'emollient' from the previous blog post below:
Moisturizers or emollients are complex mixtures of chemical agents specially designed to make the external layers of the skin (epidermis) softer and more pliable. They increase the skin's hydration (water content) by reducing evaporation. Naturally occurring skin lipids and sterols, as well as artificial or natural oils, humectants, emollients, lubricants, etc., may be part of the composition of commercial skin moisturizers. They usually are available as commercial products for cosmetic and therapeutic uses, but can also be made at home using common pharmacy ingredients.
As you can see, certain chemicals that are categorized as 'skin conditioning agents' are also 'emollients.' Confusing? Yep. Again, the idea is to return to the overall purpose of the skin firming lotion -- i.e. make the skin tighter, younger looking and moist. All of these chemicals work in concert to form the formulation at hand. Adding in various chain lengths of carbon (i.e., short chain - less viscous, long chain - very viscous) changes the properties of the product. Formulating a cream or lotion is making a mixture.
Last but not least, these chemicals will not work together over time. Spoiling might happen. Degradation of lipids, oils, and long chain molecules when the product is exposed to the atmosphere will inevitably happen. The following chemicals are incorporated to act as a preservative:
1) DMDM Hydantoin
2) Methyl Paraben
3) Propyl Paraben
Conclusions
Wow, that was a lengthy list of structures and functions -- right? Although, now you have a better
understanding of the nature of the chemicals or at least should have. Chemistry is about exploring structure and function. Especially, since the two are intricately related. In the above ingredient analysis, you were able to see how structure gives rise to play a part in function. There are gaps left in the explanation -- some of which are intentional and others which are not. Education is an ongoing process. Remember that each of you hold the power to explore the chemicals in question on your own. That is why I try (as much as possible) to stick to 'open source' without paywalls -- explanations.
In the paragraphs above, I have introduced you to the ingredients used in one product. That product was Jergen's Skin Firming Moisturizing Lotion. Since this was my first deconstruction of a product, the blog post was sort of all over the place. If you would like to see more or less of certain content, please tell me in the comments. This was a learning experience for me. In the next post, I will continue with a brief analysis of the toxicology of the ingredients in the same products. Then, I will move onto a new product which claims 'anti-aging' properties too. At that point, the ingredients which are essential will start to become completely apparent.
Until next time, Have a great day! References are below for the sources of the material above.
References:
1) EWG's Skin Deep cosmetic Database:
http://www.ewg.org/skindeep/
2) Paula's Choice (beauty product database):
http://www.paulaschoice.com/
3) Cosmetics and Toiletries.com:
http://www.cosmeticsandtoiletries.com/research/chemistry/17390254.html
4) Chemist's Corner:
http://chemistscorner.com/cosmeticsciencetalk/discussion/355/polarity-of-cosmetic-oils
5) Prospector:
http://knowledge.ulprospector.com/268/reduce-oily-feel-moisturizer-formulation/
6) Cosmetics info:
http://www.cosmeticsinfo.org/ingredient/dmdm-hydantoin
7) The Pharmaceutics and Compounding Lab (at UNC):
http://pharmlabs.unc.edu/labs/solubility/structure.htm
8) Web MD:
http://www.webmd.com/vitamins-supplements/ingredientmono-953-ashwagandha.aspx?activeingredientid=953
9) Fujimura T1, Tsukahara K, Moriwaki S, Kitahara T, Sano T, Takema Y. "Treatment of human skin with an extract of Fucus vesiculosus changes its thickness and mechanical properties." J Cosmet Sci. 2002 Jan-Feb;53(1):1-9.
10)


No comments:
Post a Comment